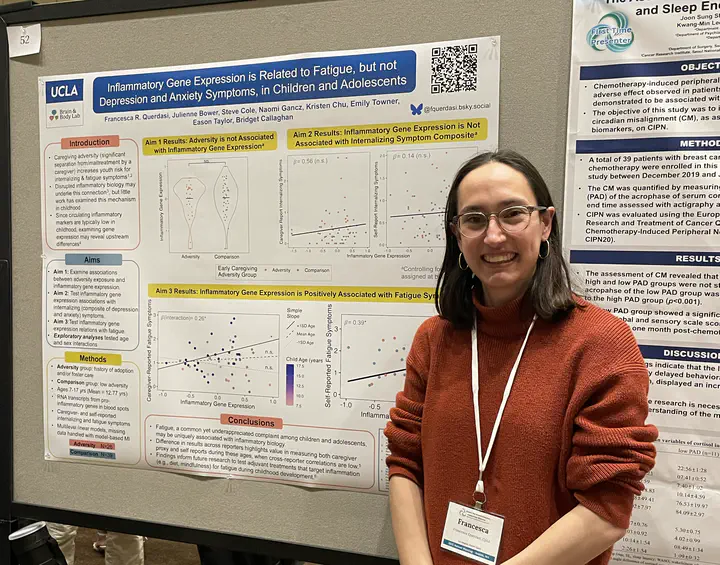
Inflammatory Gene Expression as a Mechanism Linking Early Caregiving Adversity with Childhood Internalizing Symptoms
Event: Society For Biopsychosocial Science and Medicine 2025 Annual Meeting
Location: Seattle, WA, USA
Authors: Francesca R. Querdasi, Julienne E. Bower, Steve Cole, Naomi N. Gancz, Kristen A. Chu, Emily Towner, Eason Taylor, Bridget L. Callaghan
Poster Abstract:
Early caregiving adversity (ECA), which involves interruptions and/or dysfunctions in the parent-child relationship, is associated with heightened risk for internalizing (i.e., depression and anxiety) disorders. Disrupted inflammatory biology is one mechanism thought to underlie the connection between ECA, internalizing symptoms, and fatigue, a physical symptom common across internalizing disorders. However, it remains unclear when in development links between ECA, inflammation, and internalizing and fatigue symptoms emerge. Examining inflammatory gene expression can reveal more upstream and earlier-emerging aspects of inflammatory biology in childhood, when circulating inflammatory markers are typically low.
We will examine inflammatory gene expression associations with ECA and internalizing and fatigue symptoms in a sample of N=68 children tracked across 3 annual waves (ages 6-16 years in Wave 1): N=28 with significant ECA exposure (maltreatment and/or extended parental separation), and N=40 without history of maltreatment or parental separation. Children provided dried blood spot samples for analysis of an a priori specified composite of RNA transcripts from 19 pro-inflammatory genes at one study wave, and children and caregivers completed questionnaires on children’s internalizing and fatigue symptoms at each wave. Prior analyses of this sample found that ECA-exposed children exhibited more internalizing and fatigue symptoms. In a set of pre-registered analyses (https://osf.io/fahyp), we will test (1) whether inflammatory gene expression differs according to ECA, (2) whether inflammatory gene expression is associated with caregiver-proxy or child-self reported internalizing and fatigue symptoms concurrently and one year later, and (3) explore whether inflammatory gene expression mediates the positive relationships between ECA and symptoms. Multilevel linear models, with symptoms nested within participants, will be run; missing symptom and covariate data will be handled with model-based multiple imputation. Data have been processed; analyses will be complete by February 2025.
Findings will contribute a more sophisticated understanding of how ECA shapes inflammatory health in childhood, with the potential to inform novel interventions that supplement current gold-standard treatments for internalizing symptoms in youth, which are only 40-60% effective.